This document mainly discusses the key points and strategies in process development and manufacturing (CMC) at various stages from R&D to commercialization during the “going global” process of Chinese innovative drugs. The specific content is as follows:
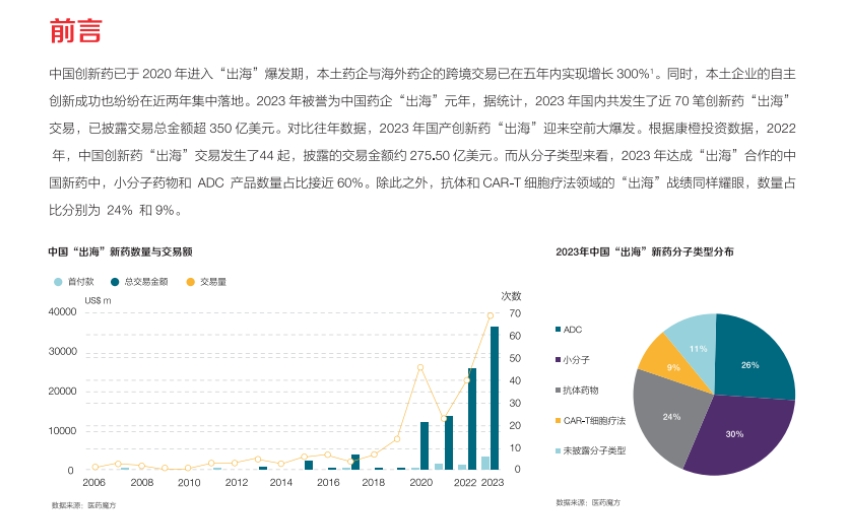
1. Current status of Chinese innovative drugs “going global”: Since 2020, Chinese innovative drugs have entered a boom period of “going global,” with 2023 being particularly prominent. The number and value of transactions have increased significantly, with small molecule drugs, ADC products, antibodies, and CAR-T cell therapies being the main types of molecules “going global.” Chinese pharmaceutical companies choose to “go global” because the domestic market size is limited; “going global” allows access to more patients, diverse regulatory frameworks, talent and technical resources, as well as diversified revenue and reduced market risk.
2. CMC strategies at each stage from new drug development to commercialization
- Preclinical research stage: It is necessary to determine CMC strategies, pharmacokinetics, etc., among which cell line development and formulation development are crucial. Cell line development can use targeted integration technology and high-throughput systems; formulation development should be based on platform process experience to determine optimal pH, etc., with the help of innovative analytical methods.
- IND application stage: When submitting a new drug clinical research application, a global perspective should be established, and neutral CMC application materials should be prepared, with a comprehensive assessment of the gaps in requirements across different jurisdictions. It is also necessary to establish complete First-in-Human (FIH) documentation and accelerate early formulation selection, such as prioritizing vials, lyophilized formulations, etc.
- Clinical research stage: The CMC R&D cycle during the clinical research stage is long and uncertain, requiring advance planning. For Phase I-II clinical trials, it is necessary to reasonably assess production needs, proactively consider production capacity, clarify formulation selection and dosage, and adopt flexible manufacturing approaches; for Phase III clinical trials, process scale-up plans and technology transfer strategies should be formulated in advance.
- Commercial-scale manufacturing stage: In the commercial manufacturing stage, analytical method validation, process characterization, and process validation are very important. Robustness should be assessed before analytical method validation; process characterization should utilize scale-down models, experimental design, etc.; process validation requires determination of batch numbers, and final submission for regulatory approval.
3. Considerations for new drug registration in specific markets: Different countries and regions have special requirements for drug registration, such as the EU requiring clarification of the responsibilities of the Qualified Person; the US requiring attention to the 356h application form and company registration listing; the UK, after Brexit, requires separate marketing authorization applications and batch testing; China has associated reviews with specific requirements for drug substance, excipients, and validation batches.
4. Summary and recommendations: Innovative drug companies aiming to go global should have a global vision at the project initiation stage and establish global talent, R&D, manufacturing, and marketing systems. Start-ups can leverage professional third-party organizations. Thermo Fisher Patheon™ can provide customers with pharmaceutical service solutions.